October 3, 2020
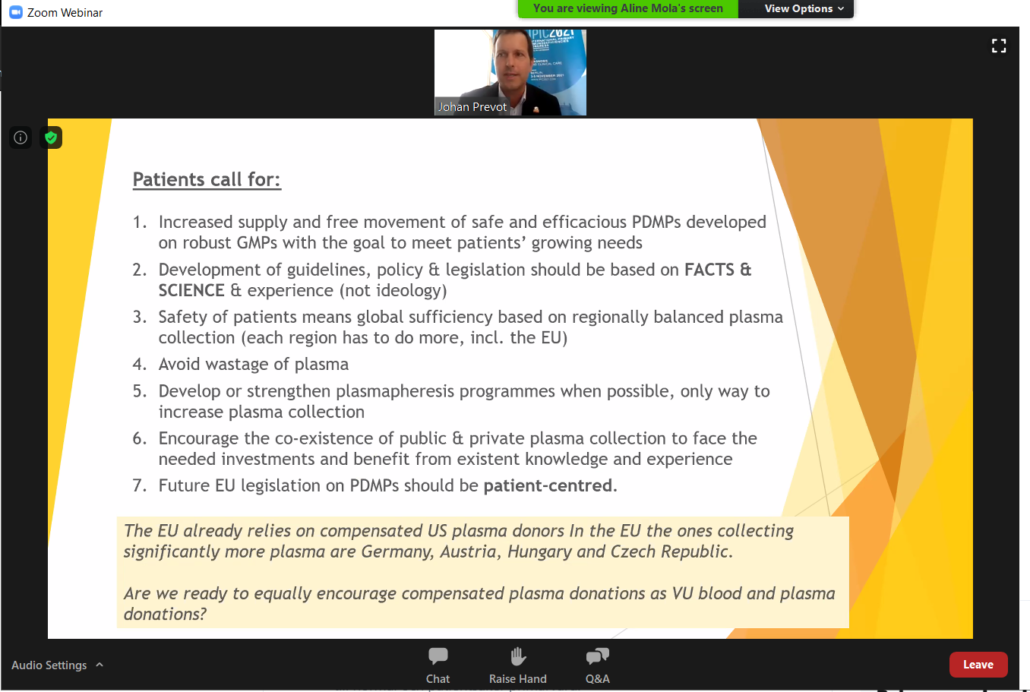
Patients with plasma disorders call for an increased collection of plasma
PLUS, the coalition of organisations representing patients with rare plasma-related disorders, amongst which IPOPI, held a virtual roundtable on “Future access to plasma-derived medicinal products: patient-centred decision making” on September 29. The event was co-organised with the support of the Member of the European Parliament, Ms Sirpa Pietikainen (EPP, Finland) and moderated by Dr Jacqueline Kerr (Paul Ehrlich Institute, Germany) and Brian O’Mahony (Irish Haemophilia Society).
Otilia Stanga, from the Romanian PID organisation (ARPID), and Johan Prevot (Executive Director of IPOPI) were the patient representatives in the panel of speakers. Other invited speakers included: Dr Guy Rautmann (representing the European Directorate for Quality of Medicines, EDQM), Dr Stefaan Van der Spiegel (from the Directorate of Health and Food Safety of the European Commission), Dr Dragoslav Domanovic (representing the European Centre for Disease Control and Prevention).
The event helped to raise awareness about the importance of sufficient plasma and blood collection for the development of plasma-derived medicinal products, key and life saving therapies for many patients with rare plasma-related disorders such as PIDs. This event was organised in light of the current and upcoming work on the European legislation on blood and plasma and aimed at ensuring that the patient’s voice was heard when looking at updating the EU legislation.
For more about PLUS:
Twitter: @PlasmaUsers
Web: www.PlasmaUsers.org